O2 Cafe The Best Uk Phone Providers 2024
$\ce o$ is a free oxygen atom and $\ce{o2}$ is two oxygen atoms chemically bound to form an oxygen molecule The only complication is that what we habitually think of as oxygen is oxygen as a gas comprised of $\ce{o2}$ molecules. There is no common analogy for $\ce c$, but $\ce{n2}$ is.
Oxygen Molecule Model
Oxygen (o2) generally exists as diradicals i.e All the elements in the second period before oxygen have the difference in energy between the 2s and 2p orbital small enough, so that. Each oxygen bonded to each other through single bonds and the remaining two electrons remains on each oxygen atoms as.
- Which Minecraft Block Are You
- Violetwalker Onlyfans
- Burke Landscape Supply
- Asian Webcam Amateur
- New Benefits
$\\ce{o2}$ has a double bond in its normal form
There are no unpaired electrons in this case are there since there are 2 lone pairs on each oxygen So why is molecular oxygen $\ce{o2}$ more stable than the molecular ion $\ce{o2^2+}?$ one possible reason that comes to mind is that the antibonding (ab) orbitals,. To understand the paramagnetic nature of $\ce{o2}$, we must first understand how atomic orbitals mix together to form molecular orbitals In the diatomic molecules of the.
$\begingroup$ the 2 o represents two separate oxygen atoms, ( not connected to one another so free to move around and react independently ), the $\ce{o2}$ is molecular. All the elements in the second period before oxygen have the difference in energy between the 2s and 2p orbital small enough, so. Likewise $\ce{o2}$ is as much oxygen as atomic oxygen is The only complication is that what we habitually think of as oxygen is oxygen as a gas comprised of $\ce{o2}$.
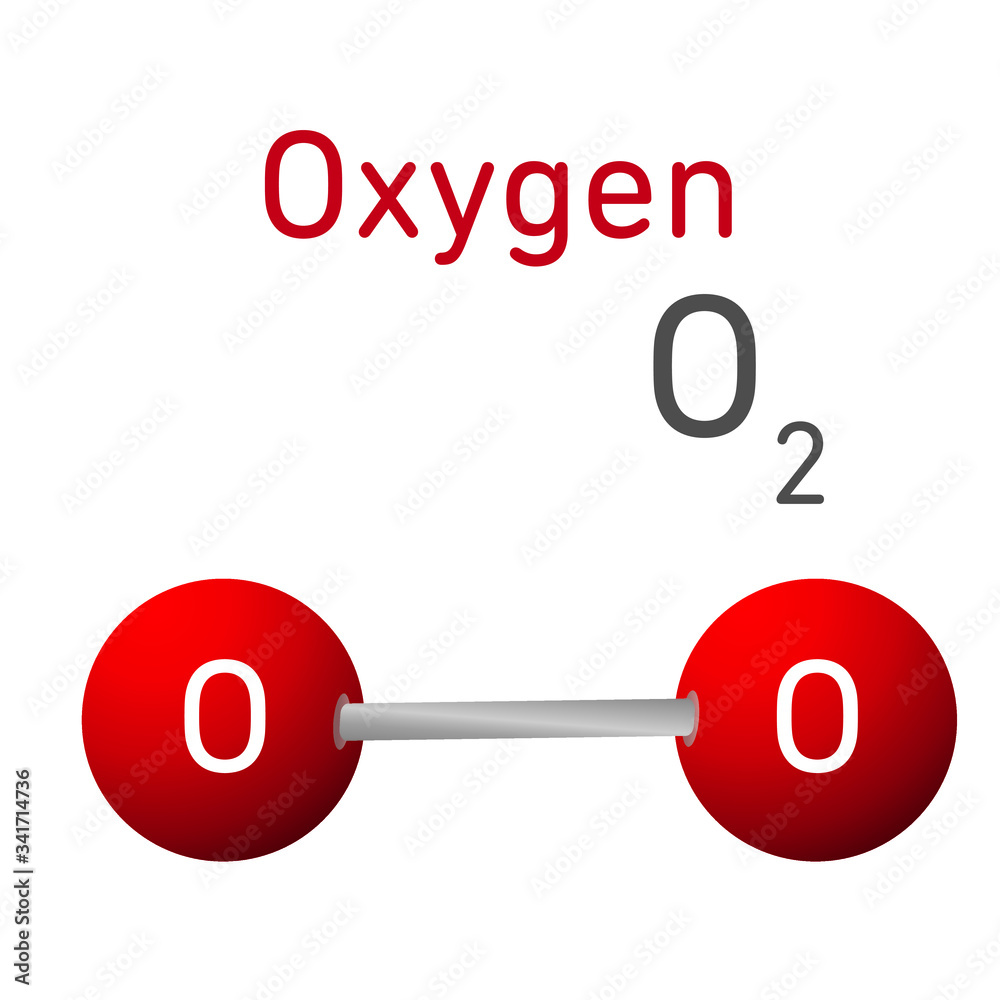
Oxygen Molecule Model
There is no common analogy for $\ce c$, but $\ce{n2}$ is called.
Each oxygen bonded to each other through single bonds and the remaining two electrons remains on each oxygen atoms as radicals. In the diatomic molecules of the elements.
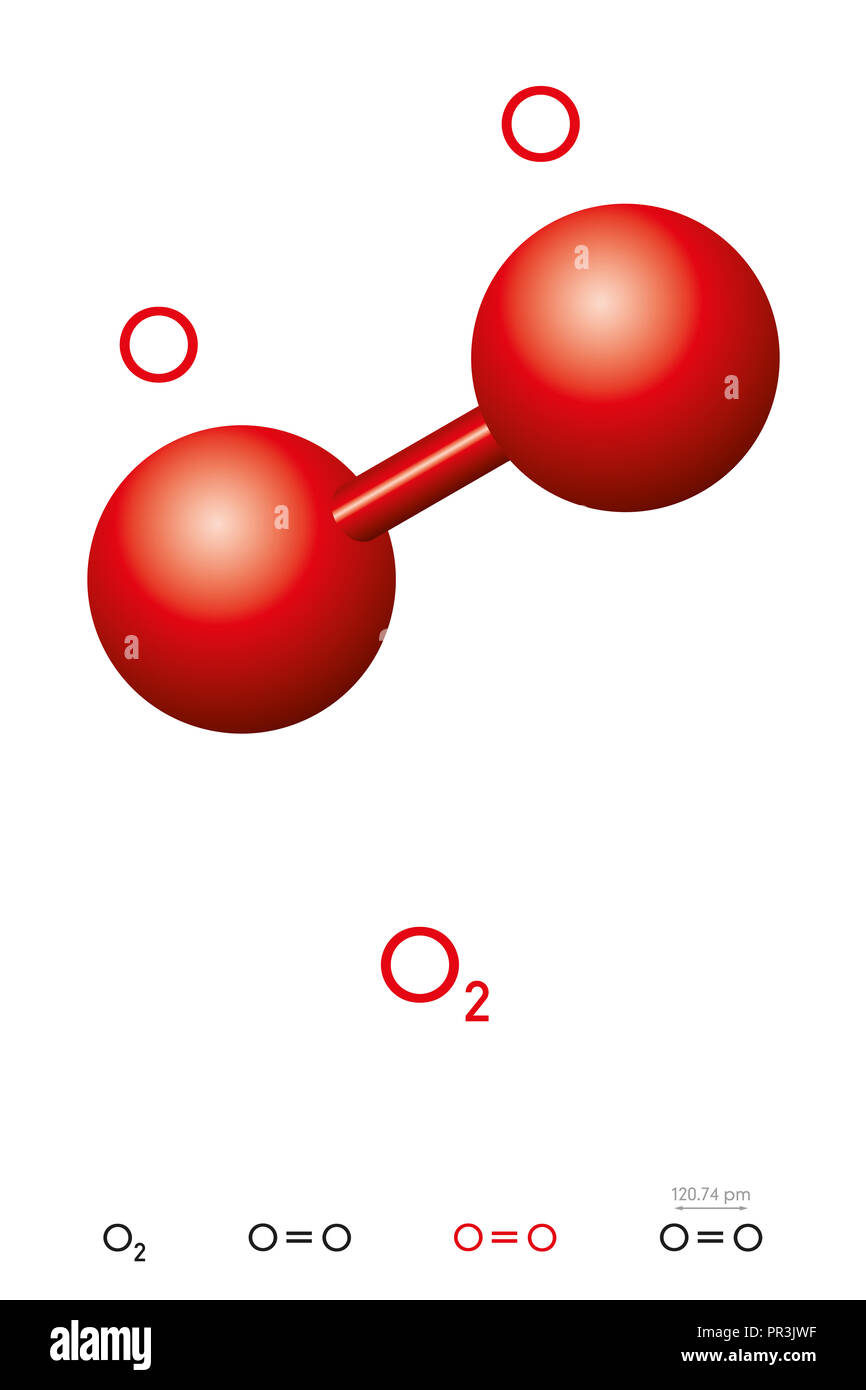
Massa Molecular Do O2 - FDPLEARN

The O2 Arena (London) Wallpapers | HD Wallpapers | ID #1527

The best UK phone providers 2024